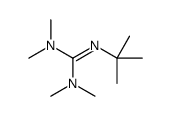
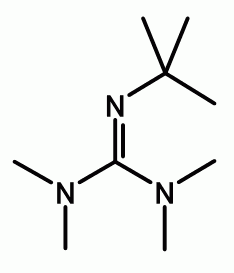
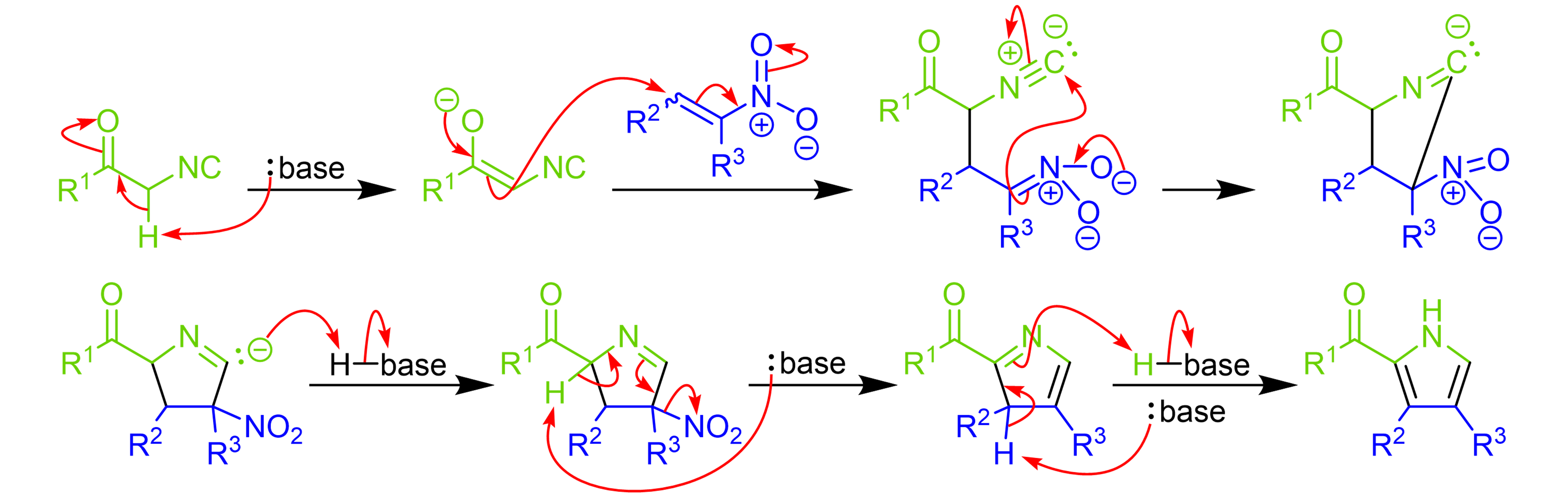
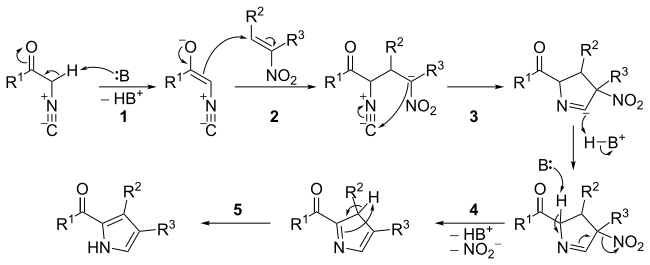
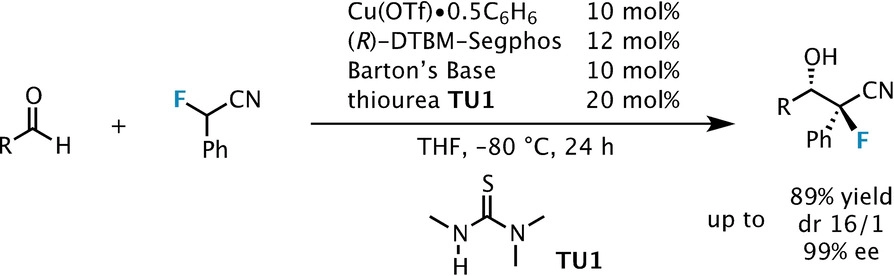
Catalytic asymmetric synthesis of CF 3 -substituted tertiary propargylic alcohols via direct aldol reaction of α-N 3 amide - Chemical Science (RSC Publishing) DOI:10.1039/C7SC00330G

Buy Bartons Silver Plated Oval Base Decorative Basket (10 cm x 6.6 cm x 9 cm, Silver) Online at Low Prices in India - Amazon.in

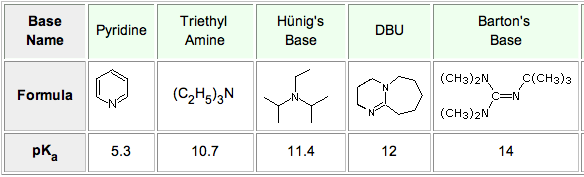
Synthesis of Highly Oxygenated Dinaphthyl Ethers via SNAr Reactions Promoted by Barton's Base | Organic Letters

Rapid Synthesis of Chiral 1,2‐Bisphosphine Derivatives through Copper(I)‐Catalyzed Asymmetric Conjugate Hydrophosphination - Yue - 2020 - Angewandte Chemie International Edition - Wiley Online Library

Brønsted Base‐Catalyzed Direct 1,6‐Conjugate Addition of Butenolide to p‐Quinone Methides | GDCh.app
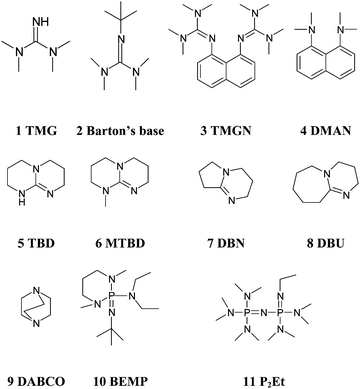
Kinetics screening of the N -alkylation of organic superbases using a continuous flow microfluidic device: basicity versus nucleophilicity - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C2OB25215E
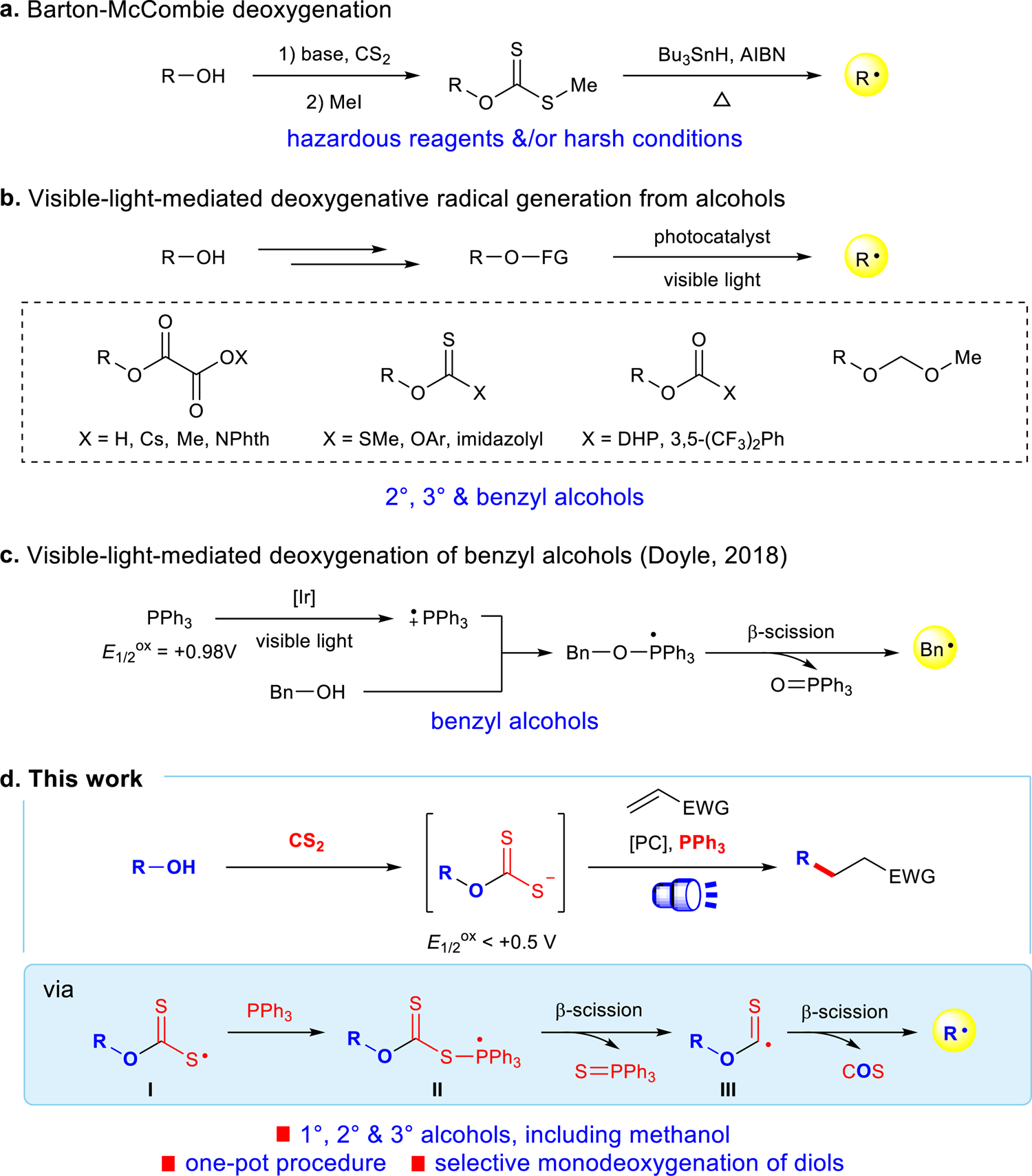
Selective deoxygenative alkylation of alcohols via photocatalytic domino radical fragmentations | Nature Communications
![Bases investigated in this study. I, diazabicyclo[5.4.0]-undec7-ene... | Download Scientific Diagram Bases investigated in this study. I, diazabicyclo[5.4.0]-undec7-ene... | Download Scientific Diagram](https://www.researchgate.net/publication/228508616/figure/fig4/AS:668842074333185@1536475785337/Bases-investigated-in-this-study-I-diazabicyclo540-undec7-ene-DBU-II-1-1-3-3.png)
Bases investigated in this study. I, diazabicyclo[5.4.0]-undec7-ene... | Download Scientific Diagram

Direct Catalytic Asymmetric Mannich-Type Reaction en Route to α‑Hydroxy-β-amino Acid Derivatives - ScienceDirect

Construction of Chiral 2,3‐Allenols through a Copper(I)‐Catalyzed Asymmetric Direct Alkynylogous Aldol Reaction - Zhong - 2020 - Angewandte Chemie International Edition - Wiley Online Library