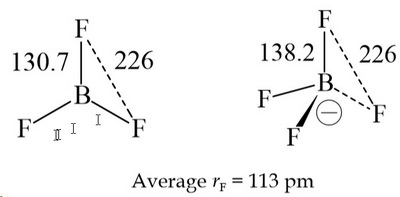
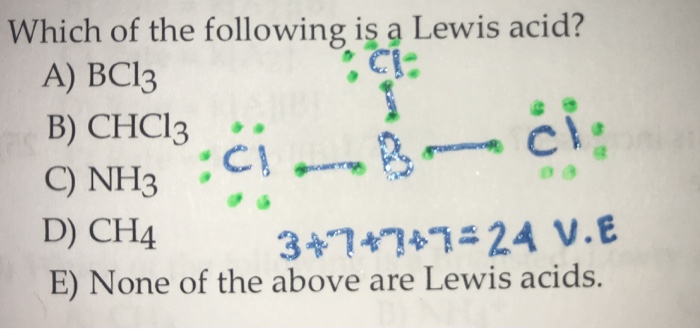
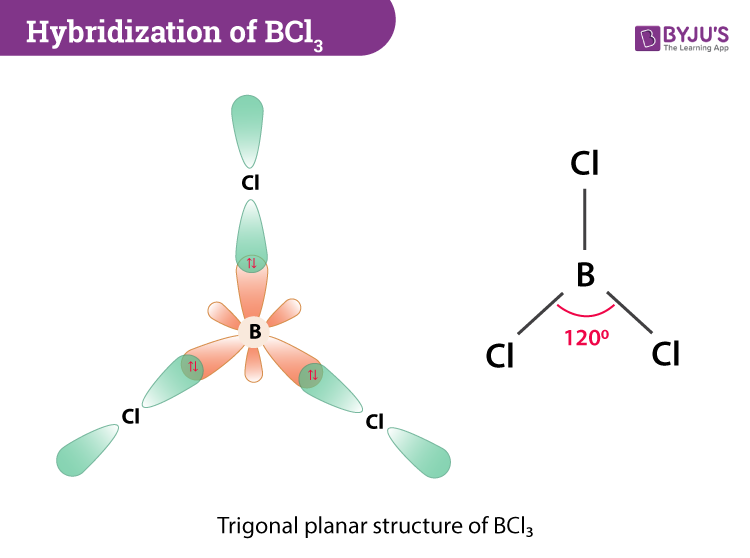
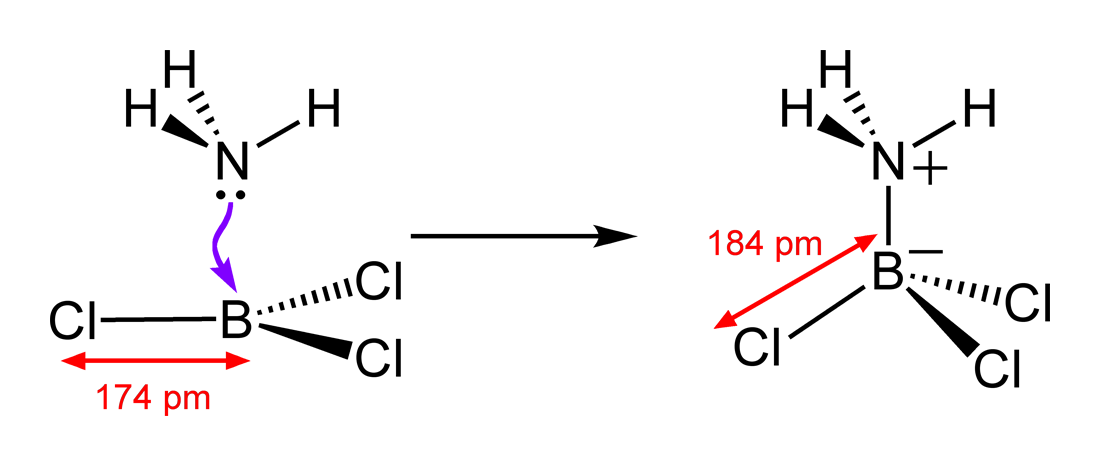
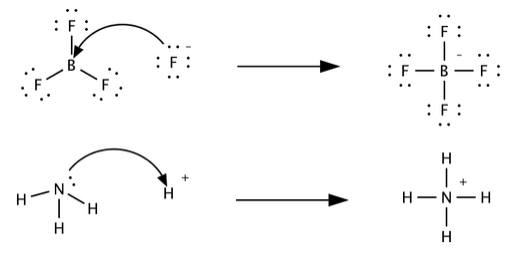
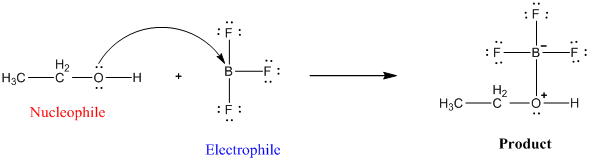
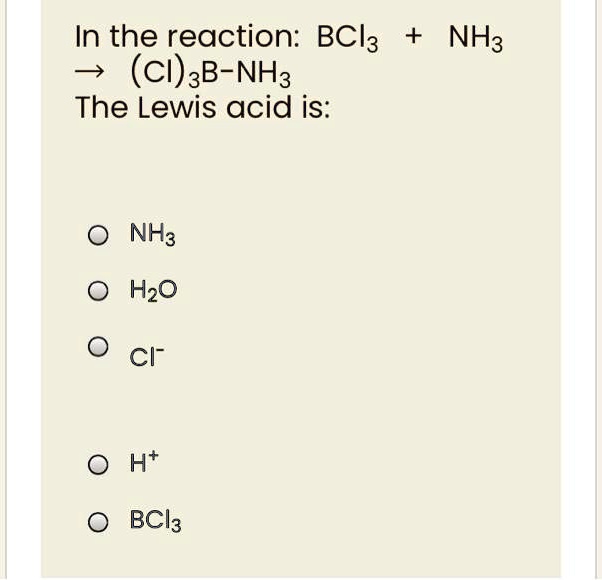
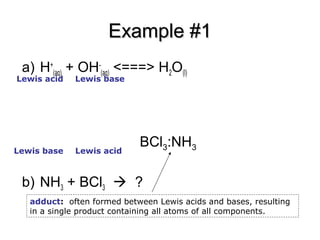
SOLVED: Identify the Lewis acids and Lewis bases: BCl3 +Ci = BCIA" The Lewis base is CI^ The Lewis acid is BCI3 SO2 + H20 = H2803 The Lewis base is H2O
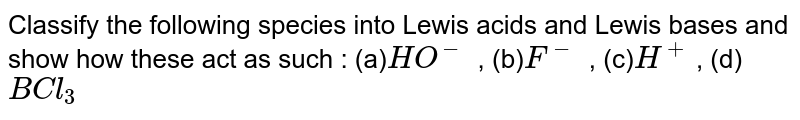
Classify the following species into Lewis acids and bases and show how these act as such: (i) BCl3 (ii) H^(+) (iii) F^(-) (iv) HO^(-)

Classify the following species into Lewis acids and Lewis bases and show how these act as Lewis ... - YouTube

Decide whether the given substance should be classified as a Lewis acid or a Lewis base.BCl3 (Hint; Draw the Lewis dot structure.) | Homework.Study.com