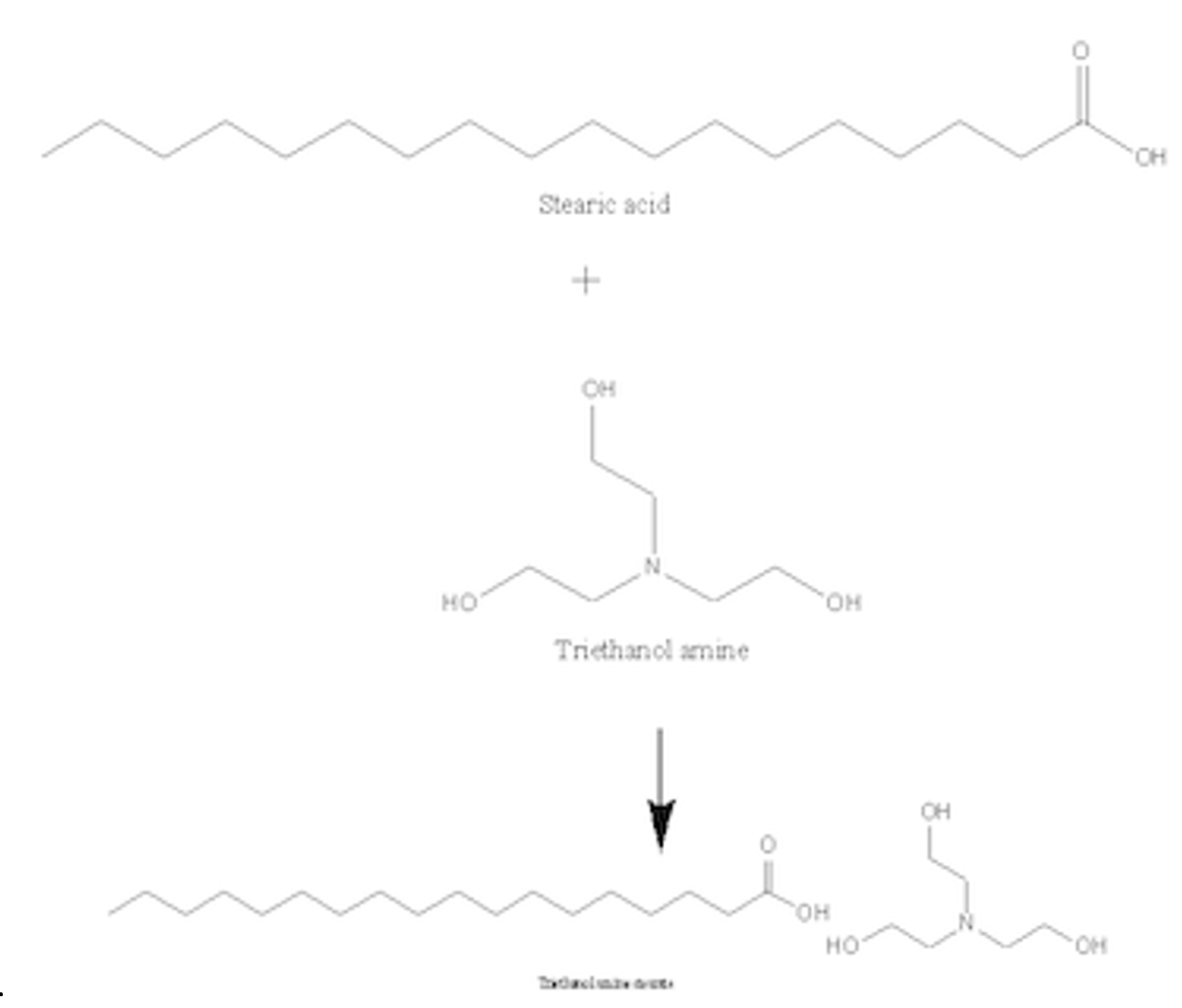
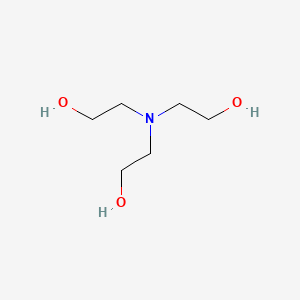
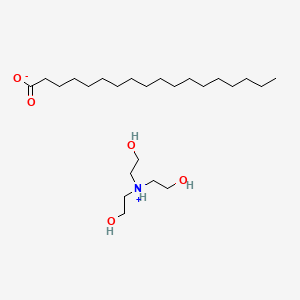
Synthesis and properties of triethanolamine-based salts with mineral and organic acids as protic ionic liquids - ScienceDirect

Write a chemical equation for the reaction that occurs between stearic acid and triethanolamine under the conditions of the experiment. How does the product of this reaction promote the formation of the

Scheme 1. Schematic view of the structures of Schiff-base ligands and... | Download Scientific Diagram

Triethanolamine as an Efficient and Reusable Base, Ligand and Reaction Medium for Phosphane‐Free Palladium‐Catalyzed Heck Reactions - Li - 2006 - European Journal of Organic Chemistry - Wiley Online Library

Unexpected Roles of Triethanolamine in the Photochemical Reduction of CO2 to Formate by Ruthenium Complexes | Journal of the American Chemical Society
Triethanolamine - The Importance Of Non-Active Ingredients In Your Skincare - The Dermatology Review

Furosemide:Triethanolamine Salt as a Strategy To Improve the Biopharmaceutical Properties and Photostability of the Drug | Crystal Growth & Design

Triethanolamine as an Efficient and Reusable Base, Ligand and Reaction Medium for Phosphane‐Free Palladium‐Catalyzed Heck Reactions - Li - 2006 - European Journal of Organic Chemistry - Wiley Online Library
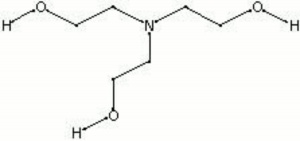

![T23040-4.0 - Triethanolamine, Free Base [2,2',2"-Nitrilothriethanol], 4 Liter T23040-4.0 - Triethanolamine, Free Base [2,2',2"-Nitrilothriethanol], 4 Liter](https://d2gdaxkudte5p.cloudfront.net/system/images/T23040-4.0_.jpg)


![T23040-1.0 - Triethanolamine, Free Base [2,2',2"-Nitrilothriethanol], 1 Liter T23040-1.0 - Triethanolamine, Free Base [2,2',2"-Nitrilothriethanol], 1 Liter](https://d2gdaxkudte5p.cloudfront.net/system/images/plabel_14881_20230721-085650.jpg)