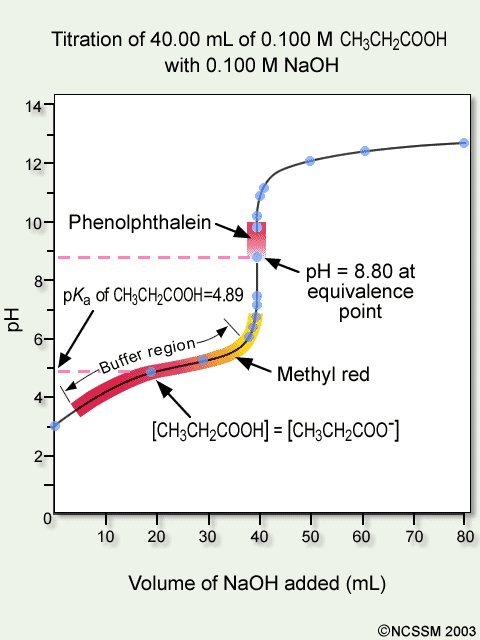
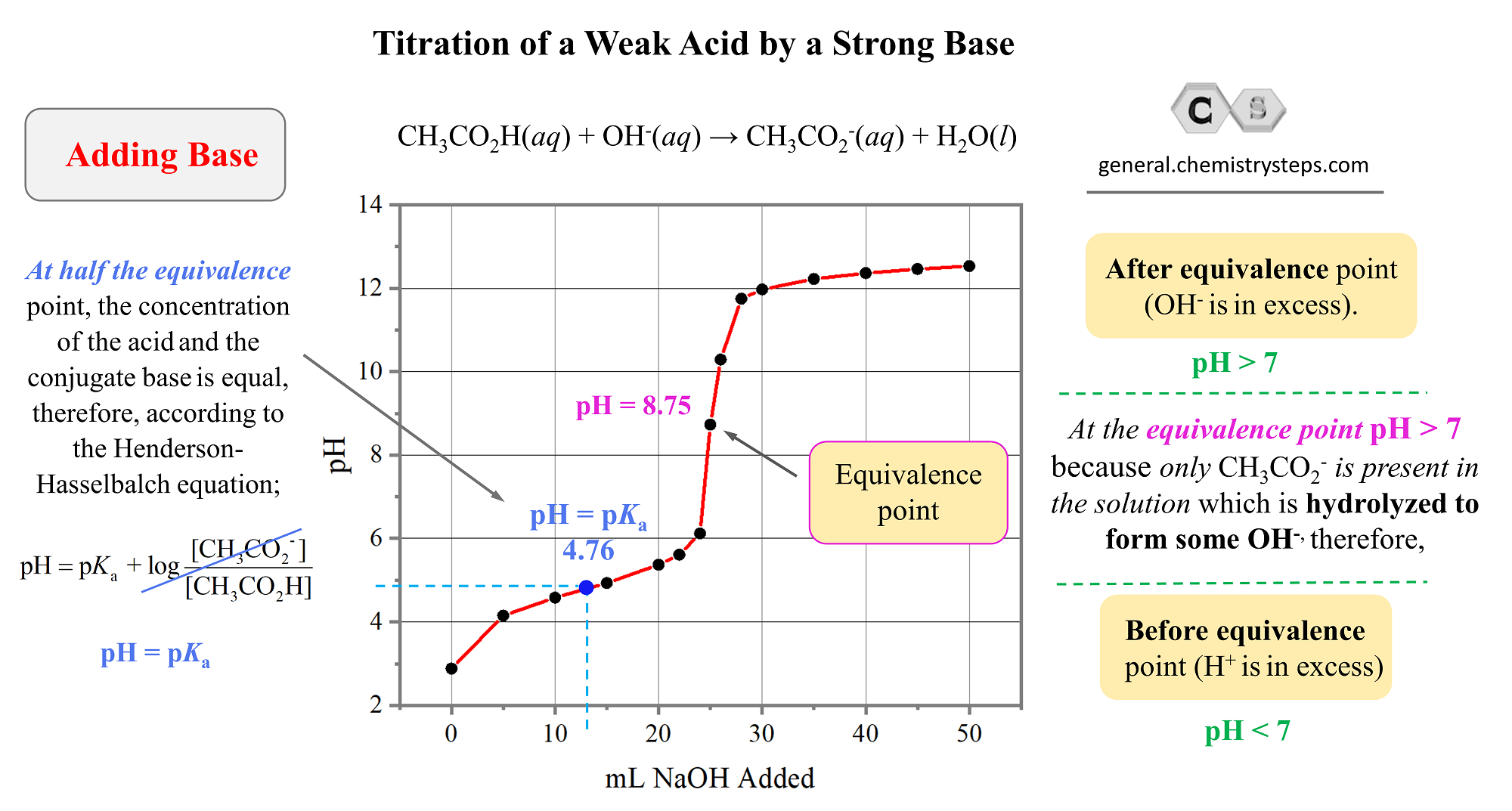
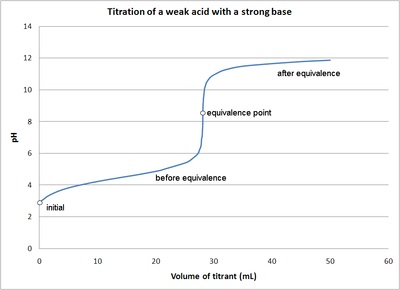
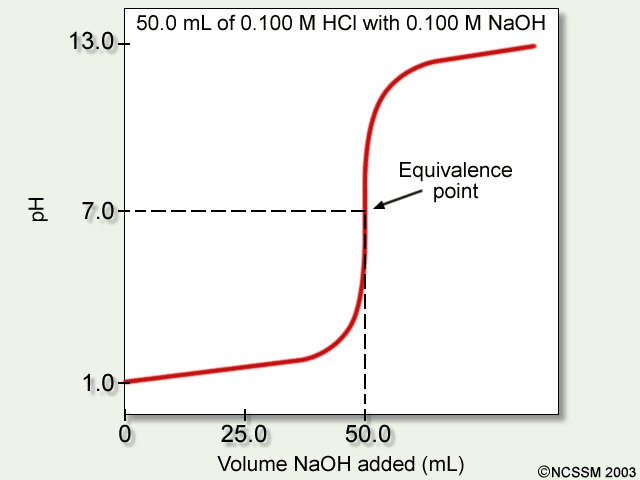
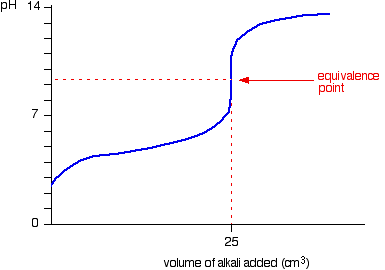
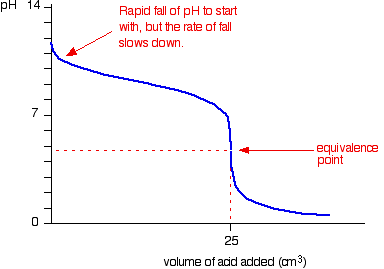
9. Investigate how pH changes for titrations of: weak acid vs. strong base | Secondary Science 4 All
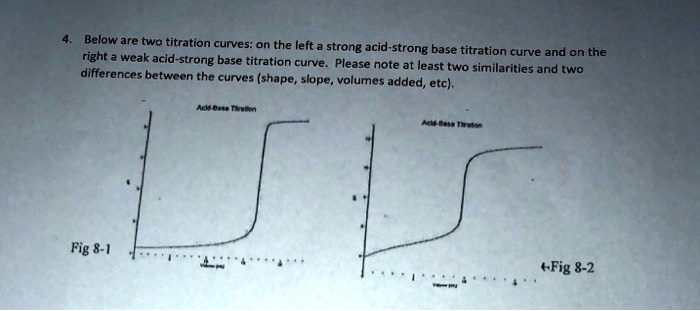
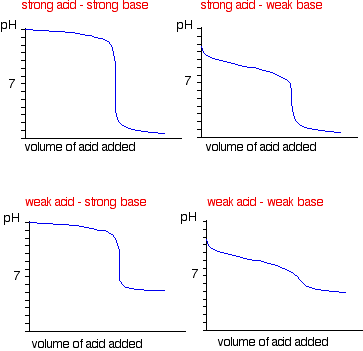
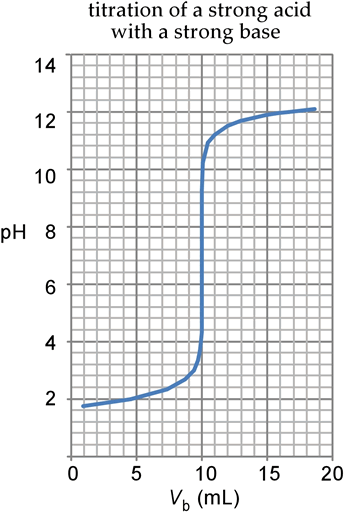
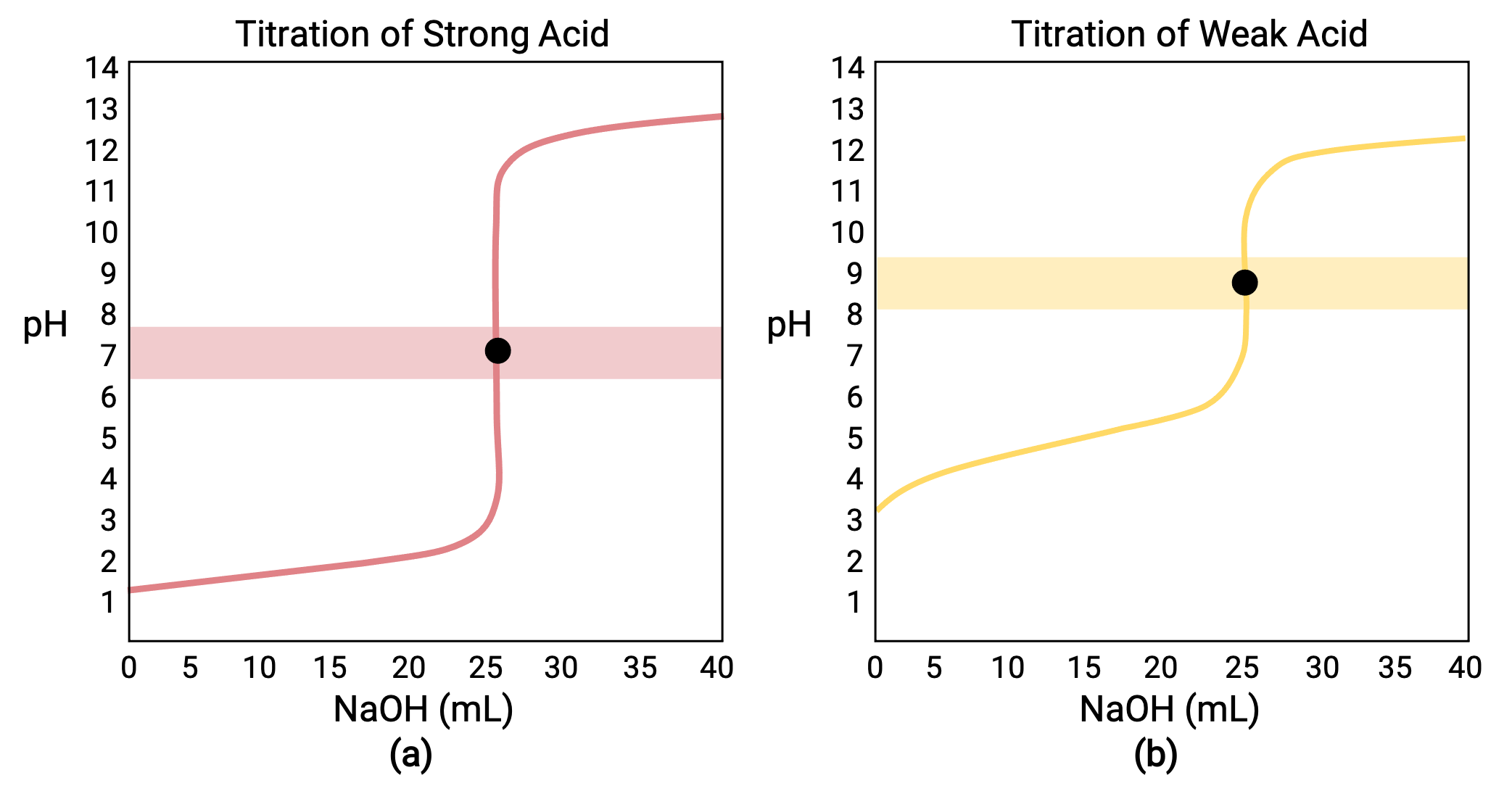
SOLVED: Below are two titration curves: on the left a strong acid-strong base right titration curve and on the weak acid-strong base titration curve. Please note at least two similarities differences between
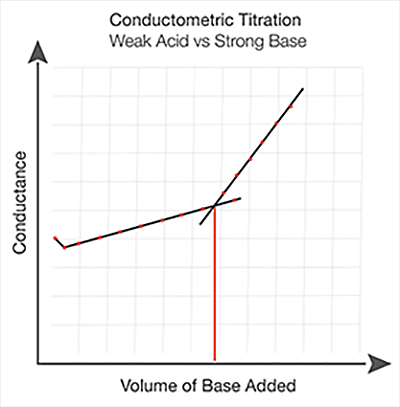
Conductometric titration of weak acid and strong base (weak acid vs strong base) - video Dailymotion

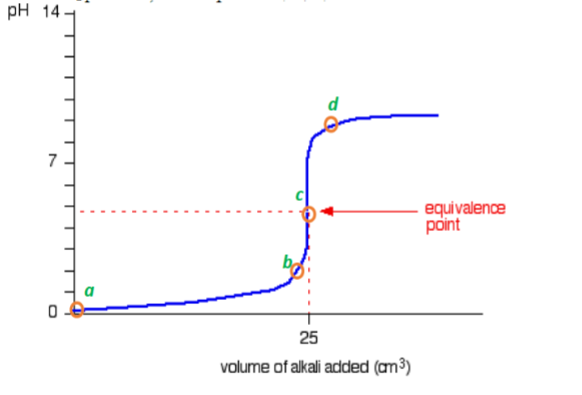
Sketch the following titration curves. a) A strong acid/strong base. b) A weak monoprotic acid/strong base. c) A weak diprotic acid/strong base. | Homework.Study.com

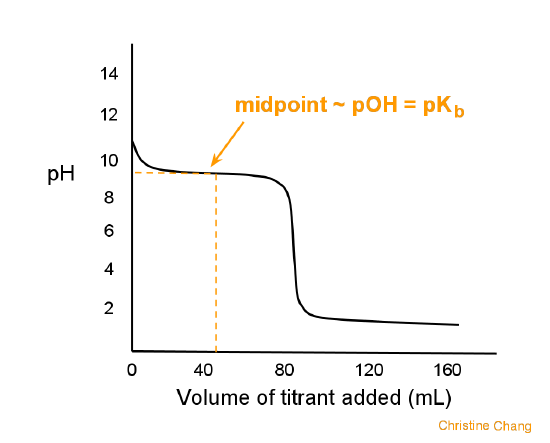
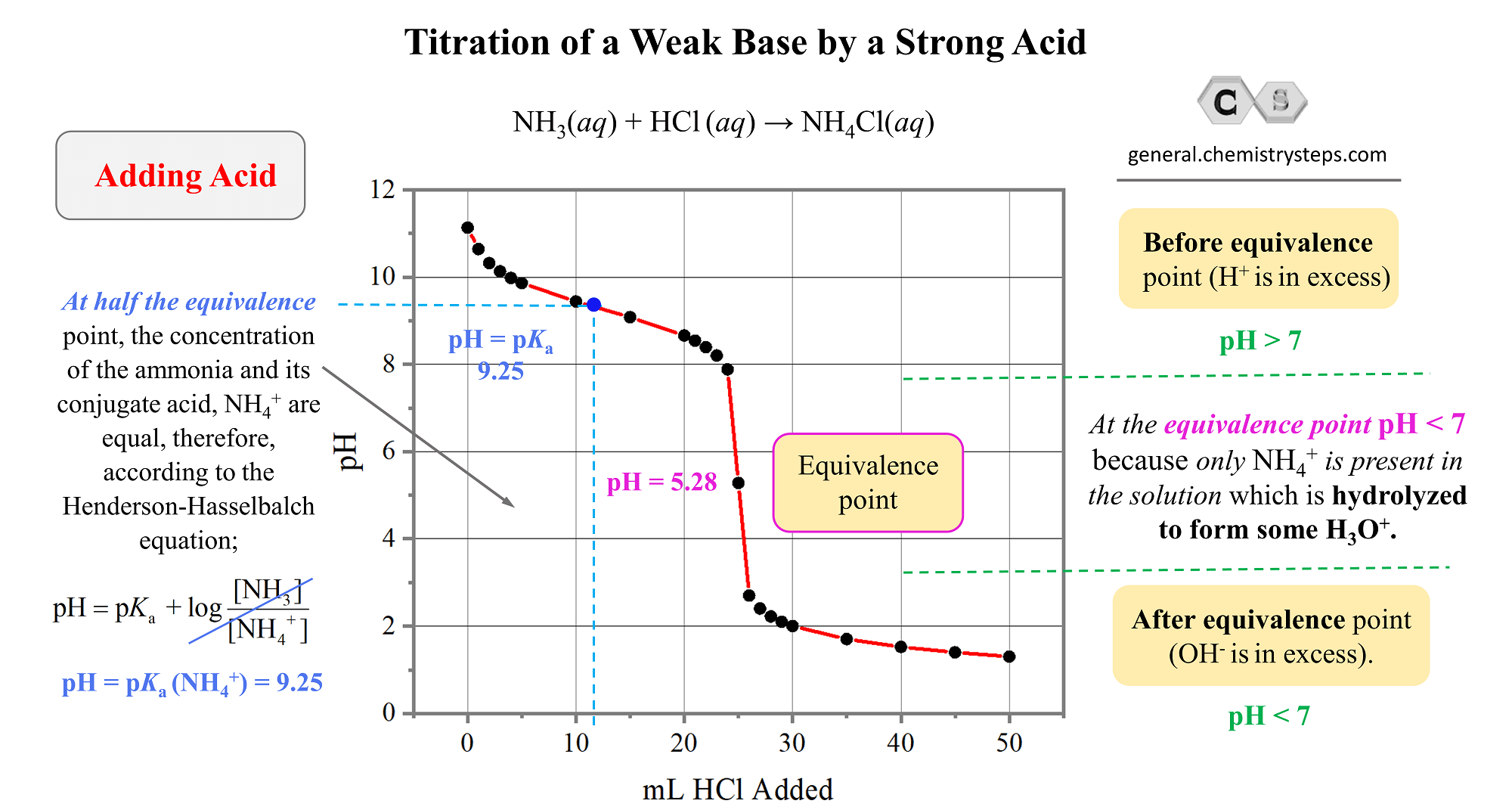
ph - What is causing the buffer region in a weak acid - strong base titration? - Chemistry Stack Exchange